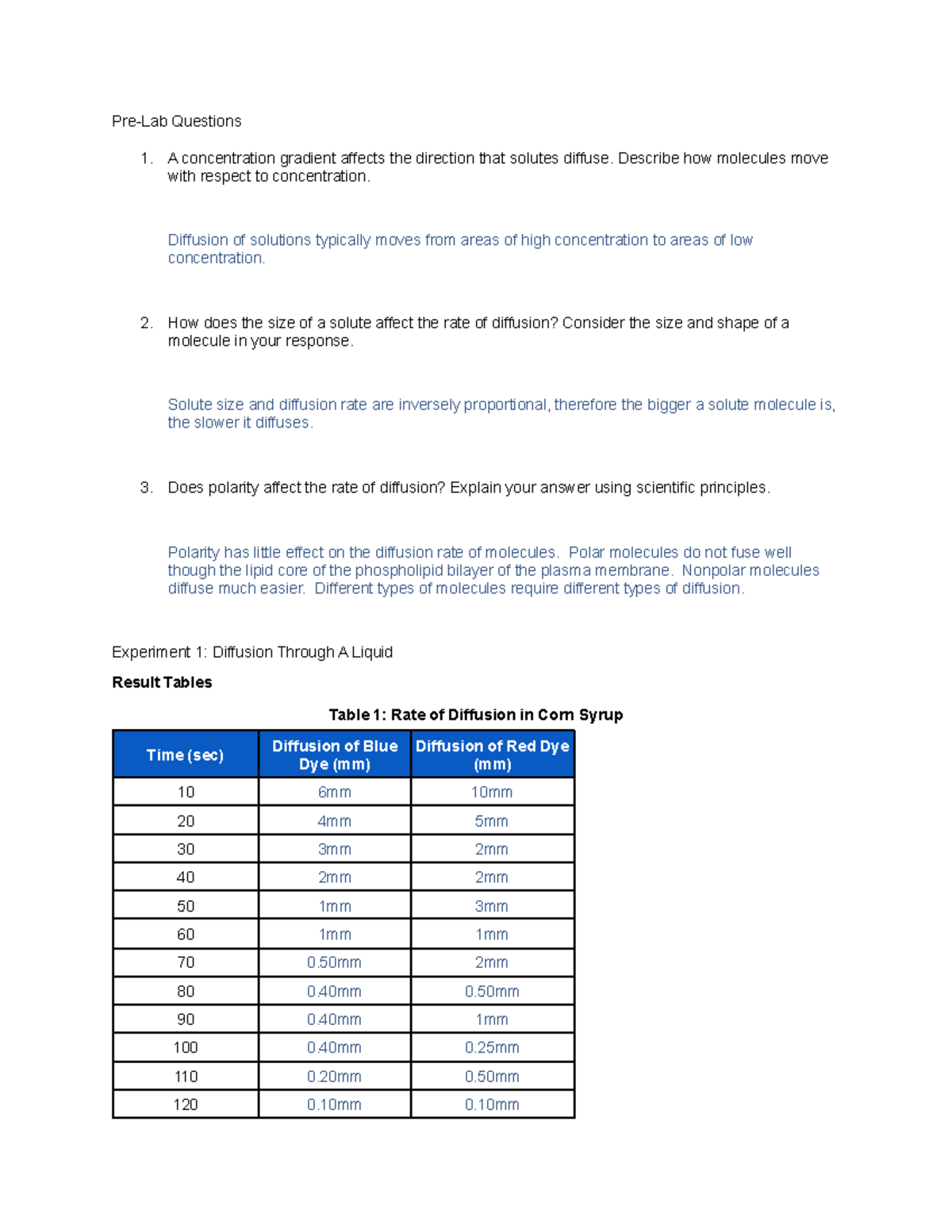
Does Polarity Affect the Rate of Diffusion
These are the factors that affect rate of Diffusion. Start your trial now.
Simple Diffusion And Passive Transport Article Khan Academy
100 4 ratings Answer As the surface area becomes larger the rate of diffusion also becomes higher because when the size of cell increases then the volume of the cell a.

. Four Things That Affect Rate of Diffusion. All other things being constant lighter weight molecules will move faster or diffuse faster that heavier molecules. Diffusion rates are dependent on molecular sizes because larger molecules diffuse slower than smaller molecules.
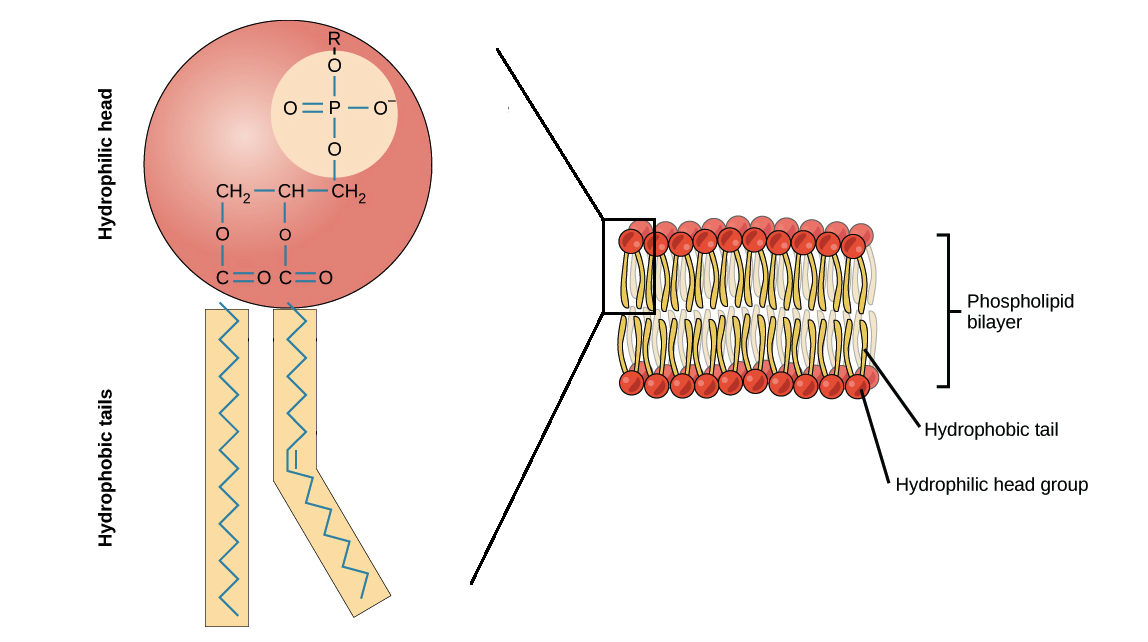
It usually happens due to a concentration gradient meaning that molecules move from an area of high concentration to an area of lower concentration. Neutral particles diffuse charged particles are. Polar molecules do not fuse well though the lipid core of the phospholipid bilayer of the plasma membrane.
Rate of diffusion is slower. Weve got the study and writing resources you need for your assignments. This is due to the fact that there is no pressure in the container and no solute present.
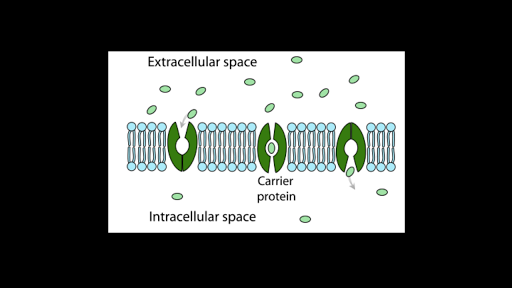
In simple diffusion molecules move freely across the membrane. First week only 499. Small polar substances diffuse.
The situation is different when molecules in solution have to pass through a cell membrane. Explain your answer using scientific principles. Start your trial now.
The polarity of molecules has little effect on their diffusion through inorganic materials. Polarity has little effect on the diffusion rate ofmolecules. So the rate of diffusion will be slower for polar molecules than non-polar molecules.
View the full answer. Does polarity affect the rate of diffusion. Diffusion Through A Liquid.
View the full answer. Molecular weight will affect the rate of diffusion. When diffusion occurs in an open system such as when spraying perfume the polarity of the molecules has no influence on the rate of diffusion.
Diffusion occurs due to the random movement of particles. We review their content and use your feedback to keep the quality high. Increased surface area - finely divided solute.
An example is shown in the image above. The size of the molecule. Different types of molecules require different types of diffusion.
The greater the concentration difference the faster the rate of diffusion. Explain your answer using scientific principles. Im not entirely sure but my best guess would be that polar molecules have stronger intermolecular bonding between them and so they are less inclined to break away from eachother and diffuse as quickly as non-polar molecules.
First week only 499. Diffusion Through Liquids Corn Syrup in mm Time sec Blue Dye mm Red Dye mm 10 20 30 40 50 60 70 80 90 100 110 120 Table 2. 1 Small non-polar molecules diffuse easily through this layer no energy is required this is passive transport.
Yes polarity affects the rate of diffusion. Experts are tested by Chegg as specialists in their subject area. Yes through passive transport non-polar molecules are able to diffuse.
Rate of Diffusion in Corn Syrup. After completing experiment 1 fill out the tables below and answer the questions following. This is mostly done by small non-polar.
Nonpolar molecules diffuse much easier. Diffusion Rate of. Temperature concentration gradient size of the molecule viscosity of the media and the distance between the two points where diffusion happens Temperature increases the kinetic energy of the molecule so it moves faster and hence the rate of diffusion increases.
Experts are tested by Chegg as specialists in their subject area. Larger particles are slower so. Solution for Does polarity affect the rate of diffusion.
Solution for Does polarity affect the rate of diffusion. Yes the polarity affects the rate of di. An open beaker of pure water has no water potential.
For example in phospholipid layer in cell membrane there are hydrophilic heads polar part of the layer and hydrophobic tails nonpolar part of the layer. But large ones will not. The sizes of the particles involved in the diffusion are important because they closely relate to the concepts of heat and energy in the context of diffusion.
Report 10 years ago. Polarity can affect the rate of diffusion. Temperature - rate proportional to kinetic energy.
Weve got the study and writing resources you need for your assignments. Like dissolves like - matching polarity. Previous question Next question.
Therefore as molar mass decreases diffusioneffusion is accelerated. When dye is added to the solution it diffuses over time. The more the concentration gradient the higher the driving force of the process and hence the.
How Does Molecular Size Affect Diffusion Rate. The smaller the molecule such as gas the faster the rate of diffusion while the larger the molecules liquid the slower the rate of diffusion. Explain your answer using scientific principles.
Does polarity affect the rate of diffusion. We review their content and use your feedback to keep the quality high.
Solubility Factors When Choosing A Solvent

Lab 6 Diffusion Pre Lab Questions A Concentration Gradient Affects The Direction That Solutes Studocu

Polarity An Overview Sciencedirect Topics

What Is A Concentration Gradient Ppt Download

How Can A Non Polar Molecule Pass Through Cell Membrane Quora

Polarity An Overview Sciencedirect Topics
Simple Diffusion And Passive Transport Article Khan Academy

Polarity An Overview Sciencedirect Topics
Escience Lab 6 Diffusion Pre Lab Questions A Concentration Gradient Affects The Direction That Studocu

Diffusion Coefficients Of Polar Organic Compounds In Agarose Hydrogel And Water And Their Use For Estimating Uptake In Passive Samplers Sciencedirect
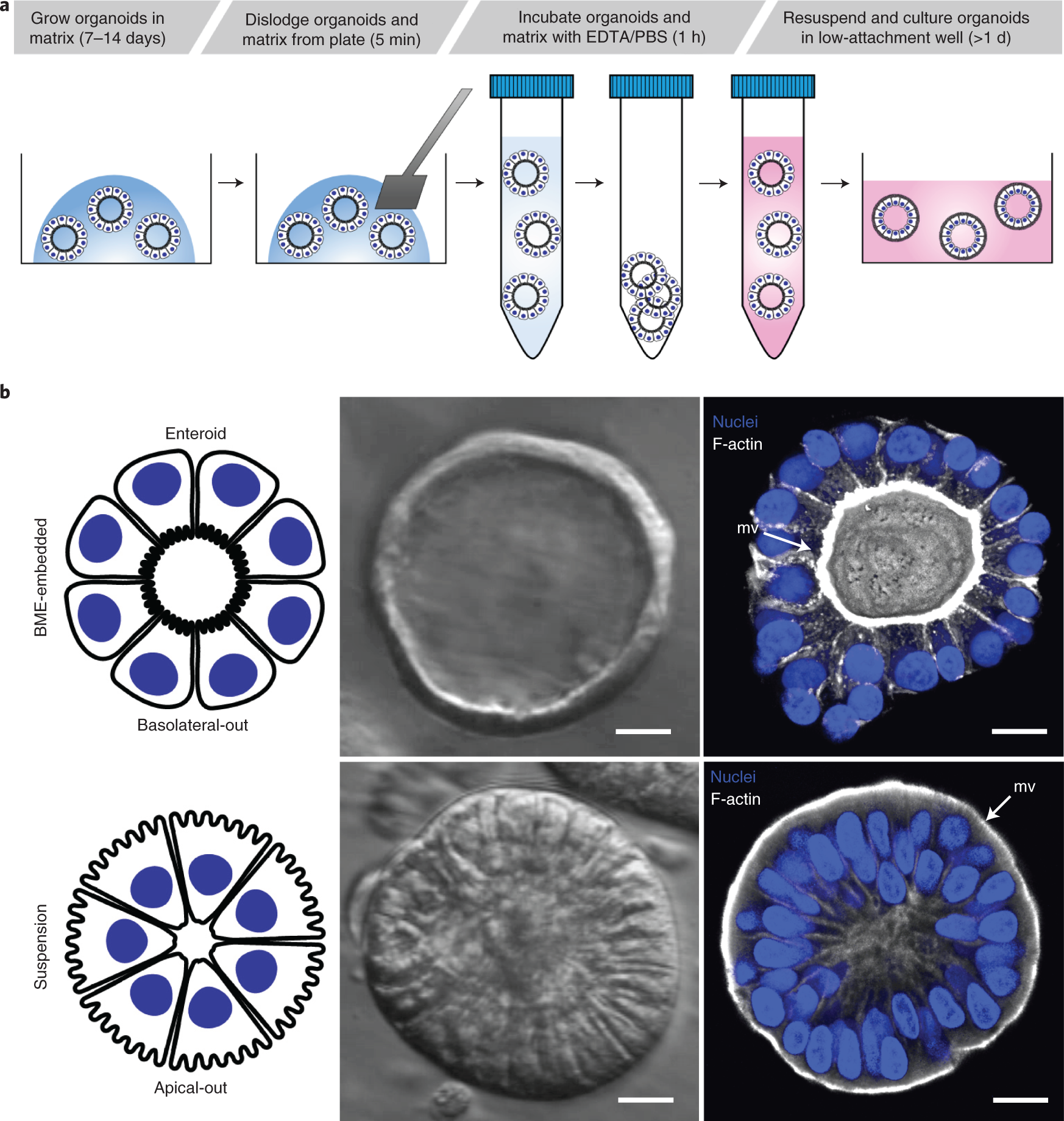
Controlling The Polarity Of Human Gastrointestinal Organoids To Investigate Epithelial Biology And Infectious Diseases Nature Protocols

Active Reading Semester Bundle Biology Textbook Series Ch 1 5 W Pdf Forms Biology Textbook Textbook Essay Questions

How To Demonstrate Diffusion Using Water Stem Little Explorers
Passive Transport Boundless Biology
How Do Water Molecules Use Osmosis To Enter Cells When Polar Molecules Can T Diffuse Through The Cell Membrane Quora

Diffusion Coefficient An Overview Sciencedirect Topics

Hhmi S Biointeractive Click And Learn Structure And Function Of Telomeres Structure And Function Telomeres Mini Lessons


Comments
Post a Comment